Paul Chin
Clinical Pharmacologist
Department of Clinical Pharmacology
Christchurch, New Zealand
Marie-Claire Morahan
Medicines Information Pharmacist
Pharmacy Department
Christchurch, New Zealand
This month we hear from Paul Chin and Marie-Claire Morahan, who recently contributed this piece to our newsletter Compass (on behalf of the Anti-infective Drugs Committee). Paul and Marie-Claire present three cases illustrating some very common challenges that arise when using dose forecasting software for vancomycin. What would you do next in these cases?
The following three cases occurred at Christchurch Hospital, where the Department of Clinical Pharmacology is the clinical lead for vancomycin TDM. In April 2020, with the onset of the pandemic, we moved to using NextDose to facilitate a more flexible workflow. We are heavily indebted to Prof Nick Holford and Dr Sam Holford for setting up our team with NextDose, and the excellent support they provide for our day-to-day clinical use of the software.
Although these cases were run on NextDose, the principle learning points are agnostic of Bayesian dose prediction software, and we hope they will be useful to the wider TDM community.
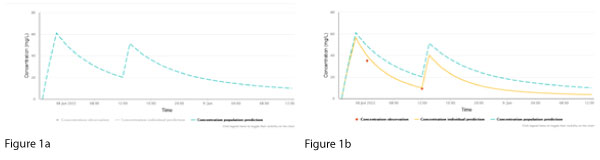
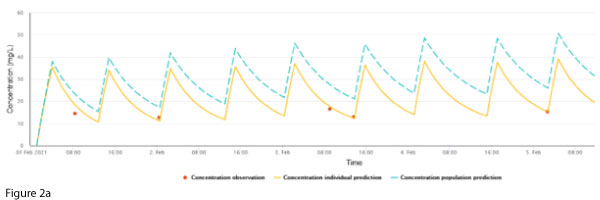
NextDose plots represent (a) a population concentration prediction, generated using the population model and the patient’s age, sex, weight, height, serum creatinine and doses (Figure 1a, dashed line, blue), and (b) the individualised prediction (Figure 1b, continuous line, yellow), generated using the measured vancomycin concentrations (Figure 1b, red dots). NextDose also calculates the maintenance dosing that is predicted to achieve the target steady-state AUC24.
Case A
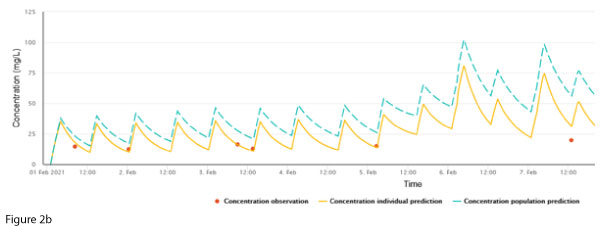
A previously well 73-year-old female was admitted following a motor vehicle accident. She had a complex left arm fracture requiring surgery and metalware. This was suspected to be infected, and elbow joint samples grew Staphylococcus epidermidis and Corynebacterium species, and vancomycin was commenced. At this time, her weight was 90 kg, height 163 cm and creatinine stable at 70 µmol/L. The patient received 1000 mg every 12 hours for an AUC24 ~ 500 mg/L.h (Figure 2a).
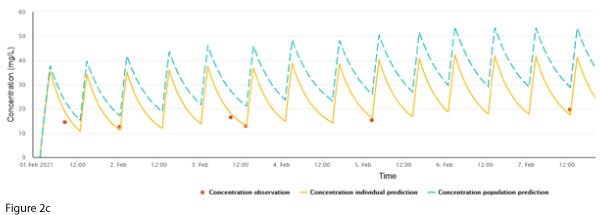
Maintenance dosing continued unchanged, however, a routine trough concentration on 7 Feb showed a discrepancy between the measured concentration and the individualised prediction (Figure 2b).
What would you do next to manage this discrepancy?
Case A Discussion:
The measured concentration is lower than the individualised prediction.
The concentration-time plot generated from Bayesian dose prediction software provides an important opportunity to ‘sense check’ the output from the software. It can be observed in Figure 2b that two of the last four plotted doses exhibit two-fold larger peaks.
A check of the data entered into the software revealed erroneous double entry for these two doses. Following correction, the discrepancy disappeared (Figure 2c).
Case B
A 63-year-old male diagnosed with acute myeloid leukaemia was commenced on chemotherapy. He developed neutropaenic sepsis with blood cultures growing Staphylococcal and Streptococcal species. Vancomycin was started, and at this time his weight was 82 kg, height 177 cm and creatinine stable at 50 µmol/L. Following feedback from concentrations, his dosing was titrated to 2000 mg every 12 hours to achieve an AUC24 ~ 600 mg/L.h (Figure 3a).
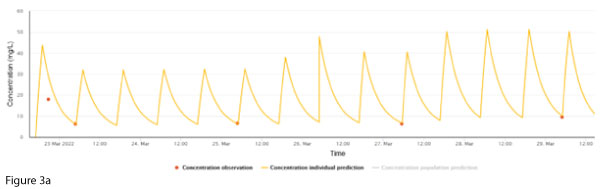
To facilitate discharge home with ongoing vancomycin, a continuous infusion of 4000 mg over 24 hours was started. A post-discharge vancomycin concentration exhibited a discrepancy between the measured concentration and the individualised prediction (Figure 3b). The creatinine taken at the same time was 49 µmol/L.
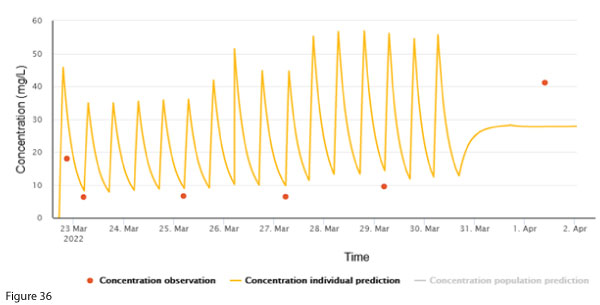
What would you do next to manage this discrepancy?
Case B Discussion:
The measured concentration is higher than the individualised prediction.
In the absence of any apparent clinical change to the patient, stable creatinine, and dosing that is expected to lead to reasonable concentrations, an error related to the most recent sample should be considered. It is unlikely that acute kidney injury (AKI) contributed to the high concentration because creatinine has a similar or shorter half-life than vancomycin1,2. Any change in vancomycin due to AKI would be reflected by a rise in creatinine &endash; but in this case it is stable.
Broadly, if there is an error, this could be due to pre-analytical (e.g. wrong patient) or analytical (e.g. assay calibration error) errors. A particular problem with vancomycin is pooling in, and adsorption to, central venous catheters, which are commonly used in the community setting to facilitate vancomycin administration3. A repeat sample from a peripheral vein would be helpful in exploring this possibility.
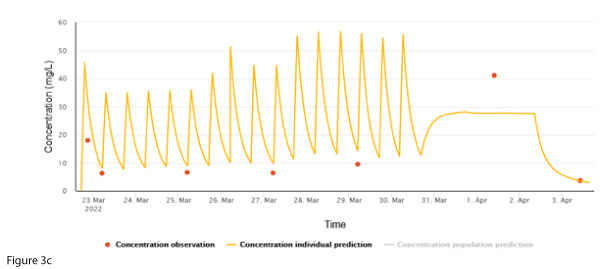
In this case, the sample was confirmed to have been derived from the patient’s central line. Due to logistic reasons (patient in community), a repeat sample was not immediately taken, and the infusion was ceased pending further investigation. The next sample was taken from a peripheral site (Figure 3c). The individual concentration prediction largely ‘ignores’ the 1 April concentration, whereas the post-cessation concentration fits well with the prediction, supporting the notion that the discrepant concentration is due to sampling from the central line.>
Case C
The following case is reproduced in NextDose but originally occurred in 2018 when the vancomycin target concentration range at our institution was 15-20 mg/L.
A 56-year-old female admitted with a cerebral aneurysm rupture subsequently had a ventricular-peritoneal shunt. The wound associated with the shunt leaked cerebrospinal fluid and grew Corynebacterium species. Vancomycin was commenced on Sep 20 and at this time her weight was 100 kg, and creatinine stable at 50 µmol/L.
She received a loading dose of 2000 mg followed by 1000 mg every 12 hours. As per guidelines at the time, a trough concentration was measured after the third dose and was found to be 21 mg/L. The creatinine was not repeated. This result was felt to be ‘close enough’ to the target trough, and dosing was continued with a plan to repeat a routine trough in two days.
Figure 4 represents a plot of the patient’s data in NextDose.
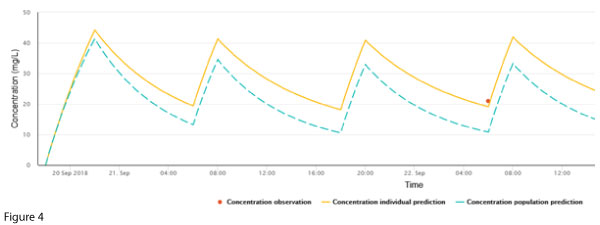
Armed with the aid of a graphical plot, what would you do next?
Case C Discussion:
The single measured concentration is double the population prediction.
While it is conceivable that the patient has worse vancomycin clearance than predicted, it is also possible that renal function declined acutely. Hence it is a matter of some urgency to check the serum creatinine and repeat the vancomycin concentration (to also assess the possibility of spurious causes of an aberrant concentration).
In our case, when the trough vancomycin was checked on 24 Sep, it was 119 mg/L. A repeat concentration corroborated this, and the creatinine was found to be 300 µmol/L. Repeated concentrations were calculated to show an elimination half-life of around 4 days. A review by the consulting renal team concluded that the cause of the AKI was multifactorial.
References:
1Kirkpatrick CM, Duffull SB, Begg EJ, Frampton C. The use of a change in gentamicin clearance as an early predictor of gentamicin-induced nephrotoxicity. Ther Drug Monit. 2003;25(5):623-30.
2Drennan PG, Begg EJ, Gardiner SJ, Kirkpatrick CMJ, Chambers ST. The dosing and monitoring of vancomycin: what is the best way forward? Int J Antimicrob Agents. 2019;53(4):401-7.
3Wright DF, Al-Sallami HS, Jackson PM, Reith DM. Falsely elevated vancomycin plasma concentrations sampled from central venous implantable catheters (portacaths). Br J Clin Pharmacol. 2010;70(5):769-72.











